views
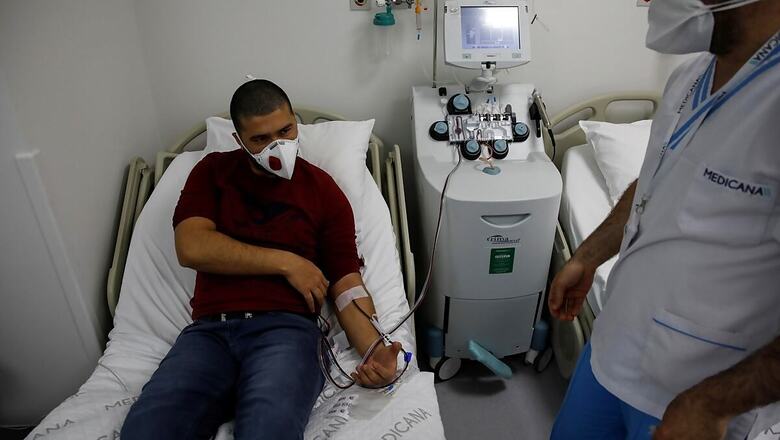
The country's apex health research body, ICMR, has issued an advisory statingthat indiscriminate use of convalescent plasma therapy (CPT) in coronavirus infected patients is not advisable. The COVID-19 caseload in India has mounted to over 89 lakh.
In an "Evidence Based Advisory to address Inappropriate Use of Convalescent Plasma in COVID-19 Patients", the Indian Council of Medical Research (ICMR) said that a potential donor for convalescent plasma should have sufficient concentration of antibody working against COVID-19. Benefits of CPT in improving clinical outcomes, reducing severity of disease, duration of hospitalisation and mortality in COVID-19 patients are dependent on the concentration of specific antibodies in convalescent plasma that could neutralise the effects of SARS-CoV-2. CPT or passive immunotherapy has been tried in the past for treatment of viral infections like H1N1, Ebola and SARS-CoV-1 etc.
The ICMR recently conducted an open label phase II multicentre randomised controlled trial in India across 39 public and private hospitals on use of convalescent plasma in the management of cases with moderate COVID-19 disease (PLACID Trial). It was concluded that CPTdid not lead to reduction in progression to severe COVID-19 or all-cause mortality in the group that received CPT as compared to the group that did not receive the therapy "PLACID is the world's largest pragmatic trial on CPT conducted in 464 moderately ill laboratory confirmed COVID-19 affected adults in real world setting wherein no benefit of use of CPT could be established," the ICMR said.
Similar studies conducted in China and the Netherlands have also documented no significant benefit of CPT in improving the clinical outcomes of hospitalised COVID-19 patients, the advisory stated. "Indiscriminate use of CPT is not advisable.It is speculated that convalescent plasma having low concentration of specific antibody against SARS-CoV-2 may be less beneficial for treating COVID-19 patients as compared to plasma with high concentration of such antibodies. "This advisory, therefore, embraces the principle that a potential donor for convalescent plasma should have sufficient concentration of antibody working against COVID-19," the advisory stated.
CPT should only be used, as advised by the ICMR for management of COVID-19 when specific criteria as mentioned are met, it stated. According to the advisory a potential donor of CPT can be a male or female, who has never been pregnant,in the age group of 18-65 years who after 14 days of symptom resolution (testing negative for COVID-19 is not necessary) can donate plasma.
Screening should be done torule out ABO incompatibility and blood borne pathogenssuch as – HIV, HBV andHCV etc and the required concentration – of IgG antibody against COVID-19 Titre of 1:640 (ELISA) and Neutralising Antibody Titres of 1:80 should be checked. For the potential recipient, ICMR said that donor can be in the early stage of COVID-19 and the therapy should be administered between 3 and 7 days from onset of symptoms, but not later than 10 days.
There should be no IgG antibody against COVID-19 by appropriate test and informed consent has to be taken. The advisoryhighlighted that presence of antibody against COVID-19 in a potential recipient makes transfusing convalescent plasma a futile intervention.
Read all the Latest News, Breaking News and Coronavirus News here

















Comments
0 comment