views
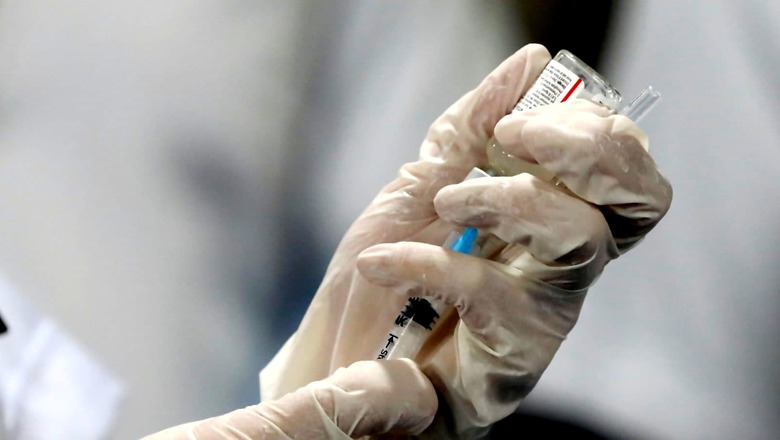
Sun Pharma on Tuesday said its subsidiary has received emergency use authorisation (EUA) from the Drugs Controller General of India (DCGI) to manufacture and market a generic version of MSD and Ridgeback’s antiviral drug molnupiravir under the brand name Molxvir in India. Earlier this year, the Mumbai-based drug major had signed a non-exclusive voluntary licensing agreement with MSD to manufacture and supply a generic version of molnupiravir in over 100 low and middle-income countries (LMICs) including India.
The DCGI has approved molnupiravir for treatment of adult patients with COVID-19 and who have high risk of progression of the disease including hospitalisation or death. Molnupiravir is an important addition to the portfolio of oral therapies available for treating Covid-19 patients, Sun Pharmaceutical Industries CEO (India Business) Kirti Ganorkar said in a statement.
“In line with our consistent efforts to accelerate access to new drugs for Covid-19 treatment, we will make Molxvir available to patients at an affordable price. We are also in the process of launching a toll-free helpline to ensure the availability of Molxvir to doctors and patients across India. Our endeavour is to make the product available in a week’s time,” he added. The recommended dose of the drug is 800 mg twice a day for five days. The duration of treatment of molnupiravir is much shorter compared to other therapies which is a significant advantage as it reduces the pill burden and enhances compliance.
Drug major Cipla and Torrent Pharmaceuticals on Tuesday also said they plan to launch antiviral drug Molnupiravir for the treatment of adult patients with COVID-19, who have a high risk of progression of the disease including hospitalization or death, under the brand name Cipmolnu.
Earlier this year, Torrent Pharma inked a non-exclusive voluntary licensing pact with MSD for manufacturing, distribution and marketing of Molnupiravir in more than 100 low-and middle-income markets, including India.
Molnupiravir has been developed by MSD and Ridgeback Biotherapeutics. It has been approved by the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) for Emergency Use Authorisation (EUA).
Read all the Latest India News here

















Comments
0 comment